
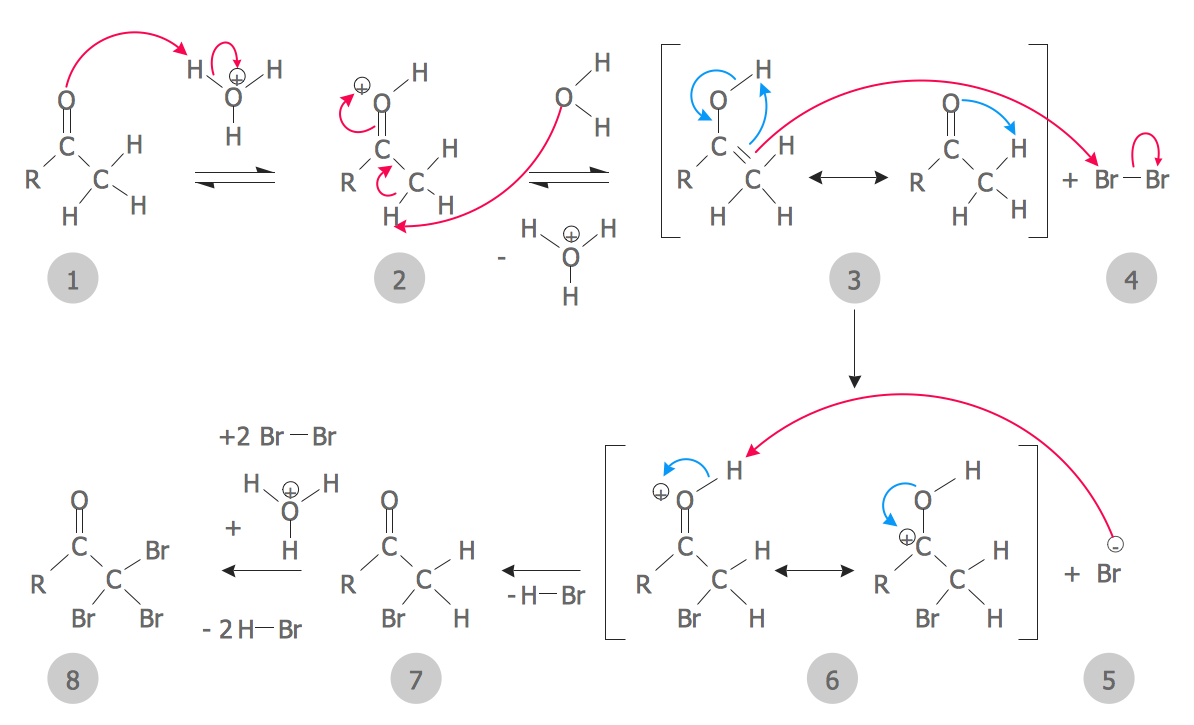
organic chemistry Mechanism of acidcatalyzed Robinson annulation
Article 20 December 2023 | Open Access Chemoselective umpolung of thiols to episulfoniums for cysteine bioconjugation Cysteine bioconjugation is an important method to modify biomolecules, but.
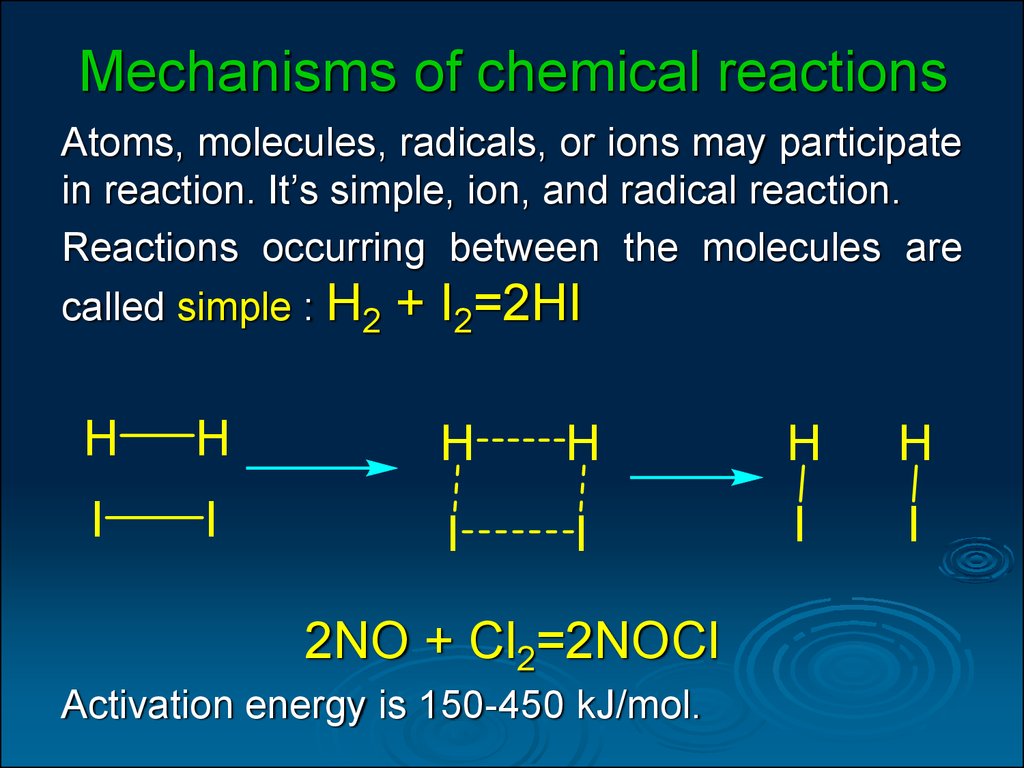
Factors affecting the rate of chemical reaction online presentation
This category includes two basic mechanisms used in chemistry to explain changes in material systems that conserve (e.g., phase change) or modify (e.g., chemical reaction) the chemical identity of the substances involved. The argumentative structure of each of these mechanisms is summarized in Table 1. These mechanisms are typically introduced.
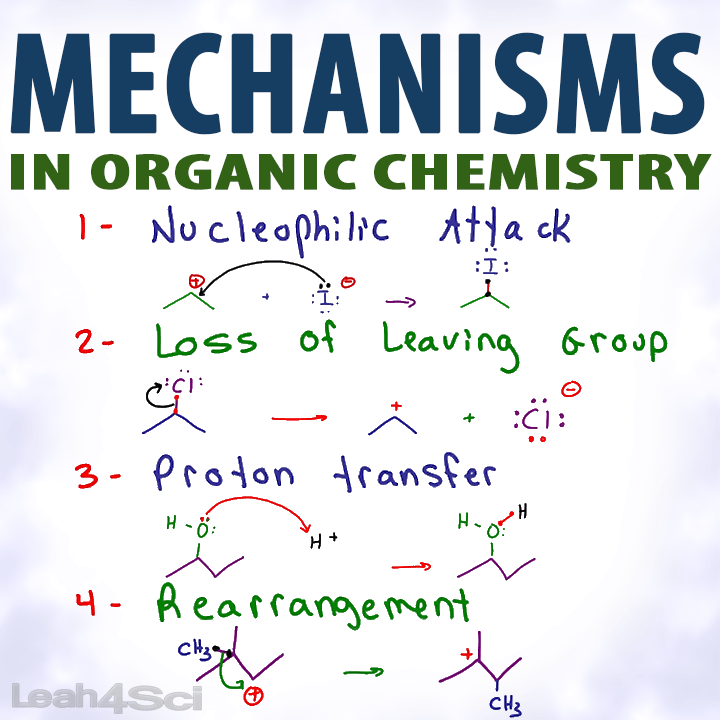
Organic Chemistry Mechanisms Tutorial Video Series by Leah4Sci
We call each step in a reaction mechanism an elementary reaction. Elementary reactions occur exactly as they are written and cannot be broken down into simpler steps. Elementary reactions add up to the overall reaction, which, for the decomposition, is: 2O3(g) 3O2(g) (4.5.2) (4.5.2) 2 O 3 ( g) 3 O 2 ( g) Notice that the oxygen atom produced in.
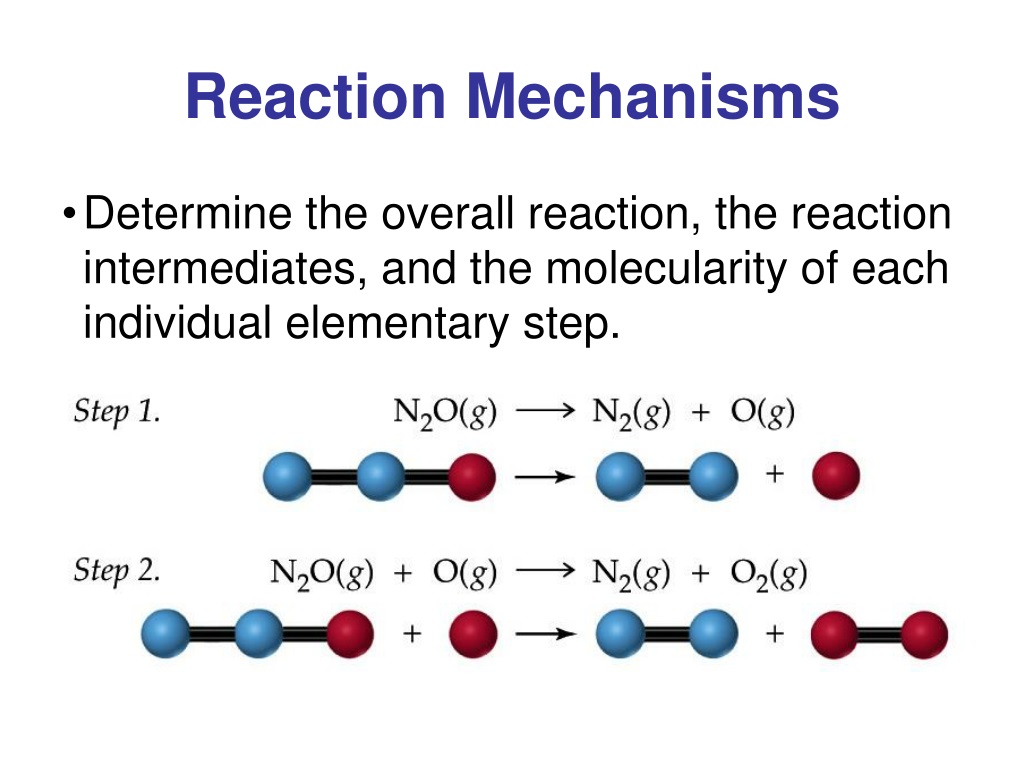
PPT Reaction Mechanisms PowerPoint Presentation, free download ID
RMG is an automatic chemical reaction mechanism generator that constructs kinetic models composed of elementary chemical reaction steps using a general understanding of how molecules react. Flux diagram for the pyrolysis of 1,3-hexadiene, an example model generated with RMG, showing the net carbon flux at an instant near the end of the.

Reaction Mechanisms Overview ( Video ) Chemistry CK12 Foundation
The reaction mechanism (or reaction path) provides details regarding the precise, step-by-step process by which a reaction occurs. The decomposition of ozone, for example, appears to follow a mechanism with two steps: O3(g) O2(g) + O O +O3(g) 2O2(g) O 3 ( g) O 2 ( g) + O O + O 3 ( g) 2 O 2 ( g)
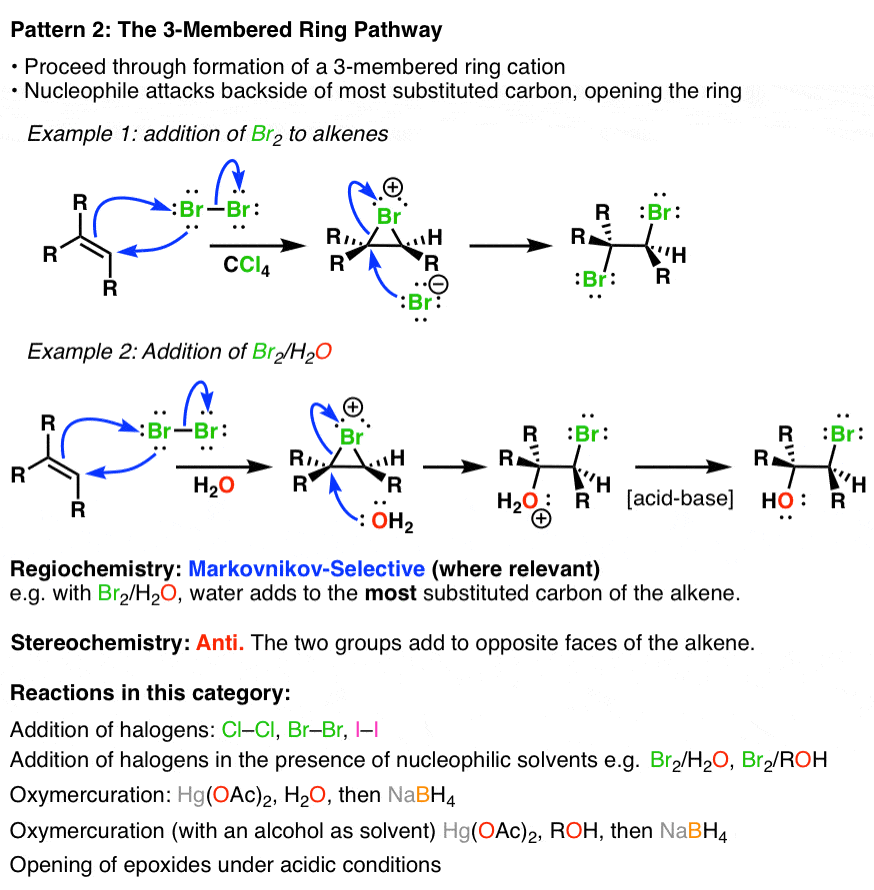
Summary Alkene Reaction Pathways — Master Organic Chemistry
The reaction mechanism (or reaction path) provides details regarding the precise, step-by-step process by which a reaction occurs. The decomposition of ozone, for example, appears to follow a mechanism with two steps: O3(g) → O2(g)+O O+O3(g) → 2O2(g) O 3 ( g) → O 2 ( g) + O O + O 3 ( g) → 2 O 2 ( g)
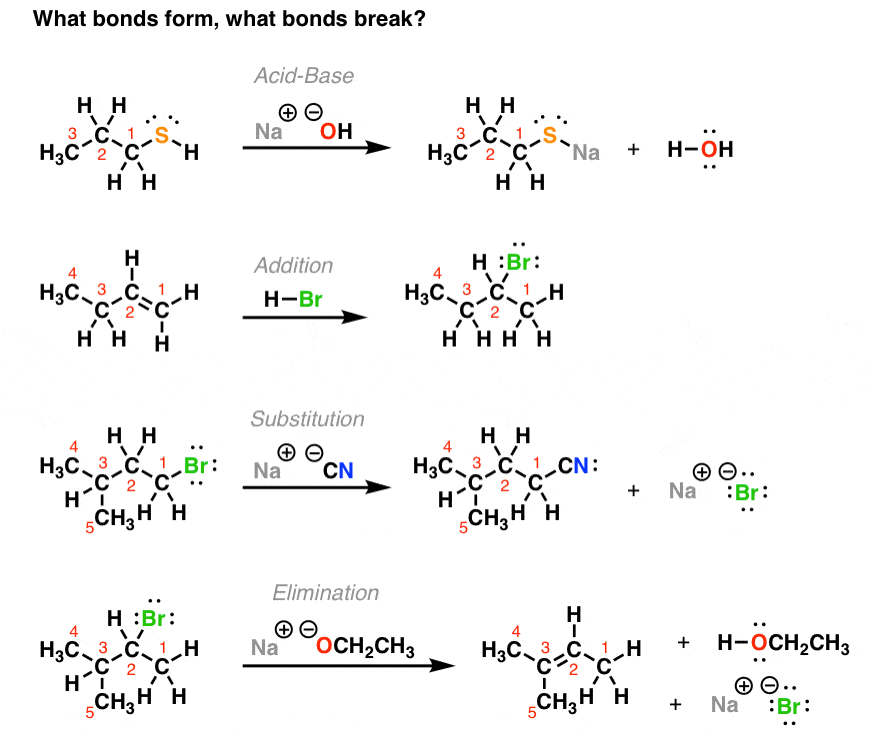
The 4 Major Classes of Reactions in Org 1 Master Organic Chemistry
However, some unimolecular reactions may be the only step of a single-step reaction mechanism. (In other words, an "overall" reaction may also be an elementary reaction in some cases.) For example, the gas-phase decomposition of cyclobutane, C 4 H 8 , to ethylene, C 2 H 4 , is represented by the following chemical equation:
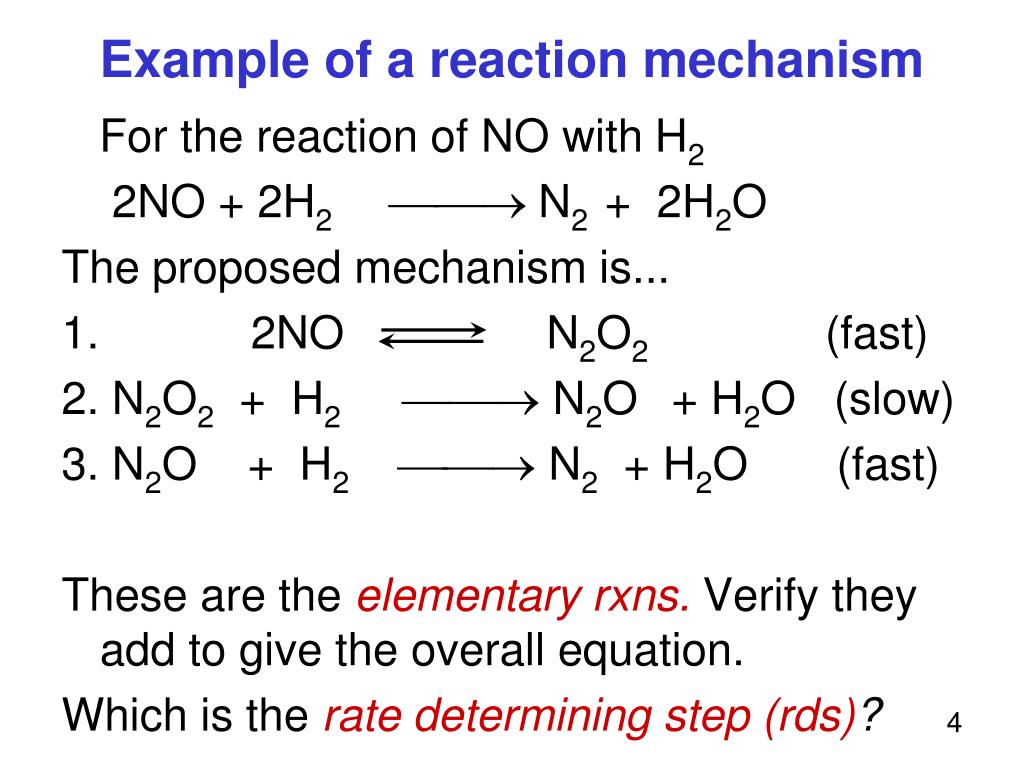
PPT Part V Reaction Mechanisms PowerPoint Presentation
Reaction mechanisms | Organic Chemistry 1: An open textbook 7.5. Reaction mechanisms An acid-base (proton transfer) reaction For our first example of chemical reactivity, let's look at a very simple reaction that occurs between hydroxide ion and hydrochloric acid: HCl+OH− → H2O+Cl- (6.1.1) (6.1.1) H C l + O H − → H 2 O + C l -

Organic Chemistry Reaction Mechanism Pattern Examples YouTube
Chemical reactions, Kinetic analysis, Order, Reaction mechanisms, Students Abstract An introductory guide to deducing the mechanism of chemical reactions is presented. Following a typical workflow for probing reaction mechanism, the guide introduces a wide range of kinetic and mechanistic tools.
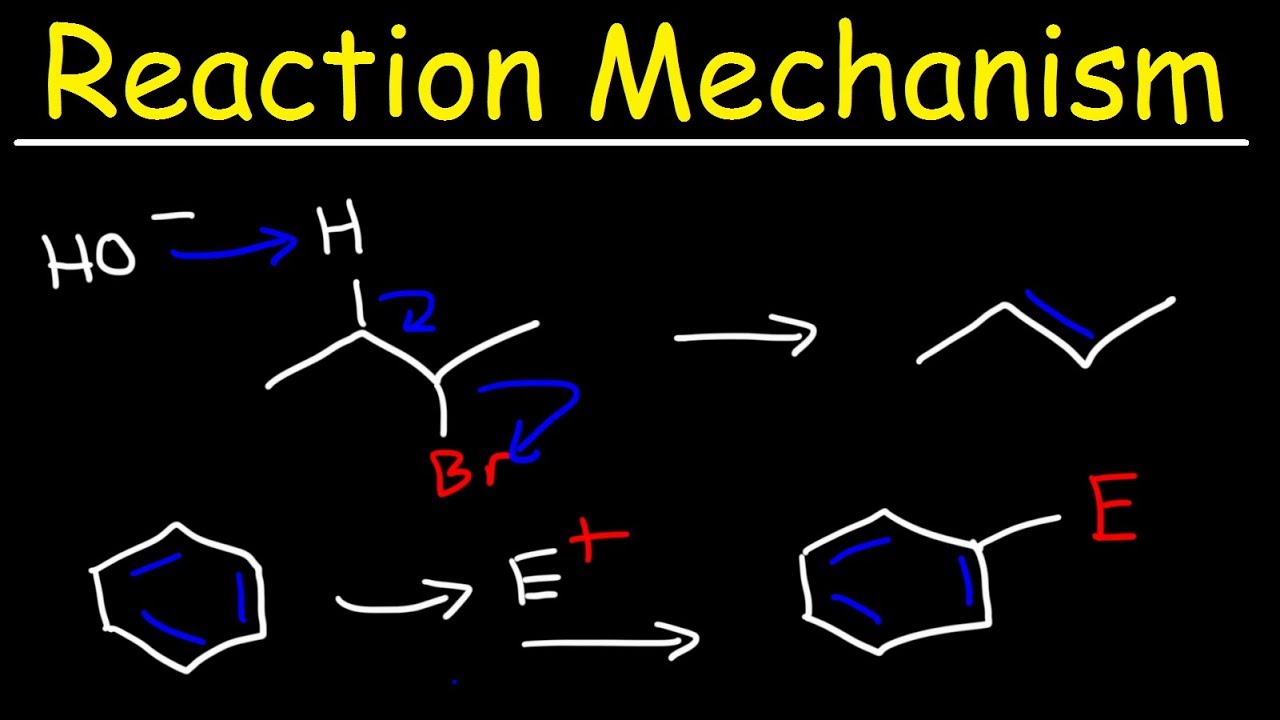
Organic Chemistry Reaction Mechanisms Addition, Elimination
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs. [1] A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases.

The reaction mechanism of sulfuric acid with polyethylene, introducing
A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. A reaction that occurs in two or more elementary steps is called a multistep or complex reaction. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step.
Chemistry Equation Symbols
The mechanism of a chemical reaction is the sequence of actual events that take place as reactant molecules are converted into products. Each of these events constitutes an elementary step that can be represented as a coming-together of discrete particles ("collison") or as the breaking-up of a molecule ("dissociation") into simpler units. The molecular entity that emerges from each step may.

chemistry world E1 REACTION MECHANISM & EXAMPLES
Chem1 (Lower) 17: Chemical Kinetics and Dynamics 17.4: Reaction Mechanisms
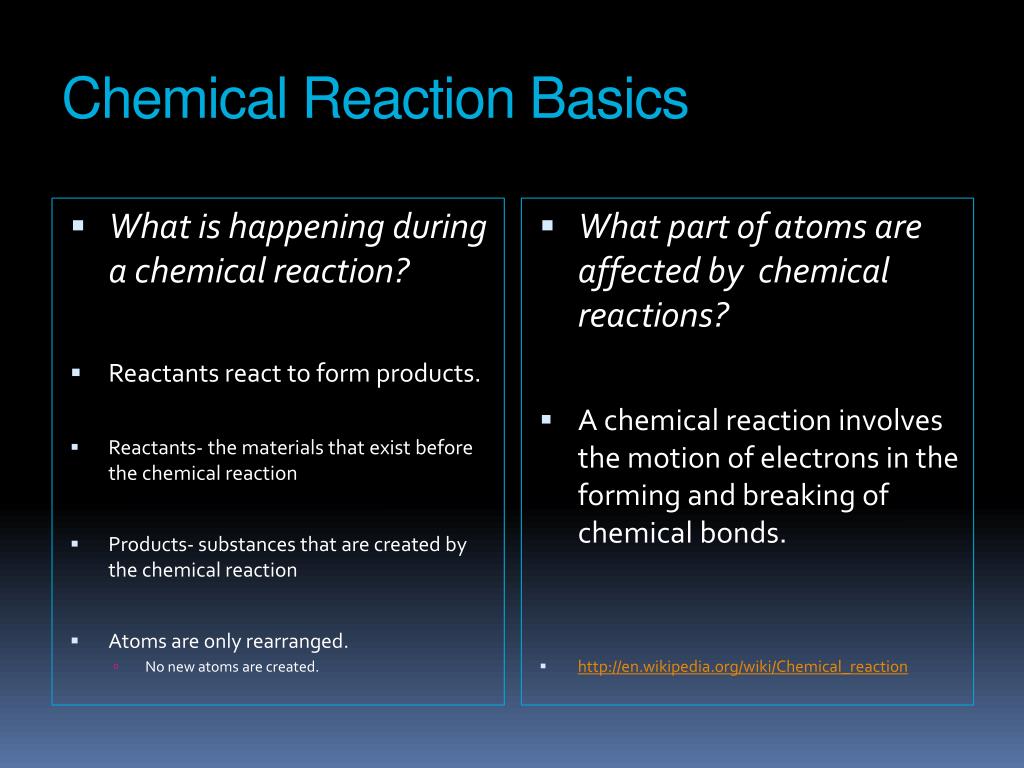
PPT Chemical Reactions (Chemical Rx) PowerPoint Presentation, free
The general rate law for a unimolecular elementary reaction (A → products) is. rate = k[A]. r a t e = k [ A]. For bimolecular reactions, the reaction rate depends on the number of collisions per unit time, which is proportional to the product of the concentrations of the reactants, as shown in Figur e 14.6.1 14.6. 1.
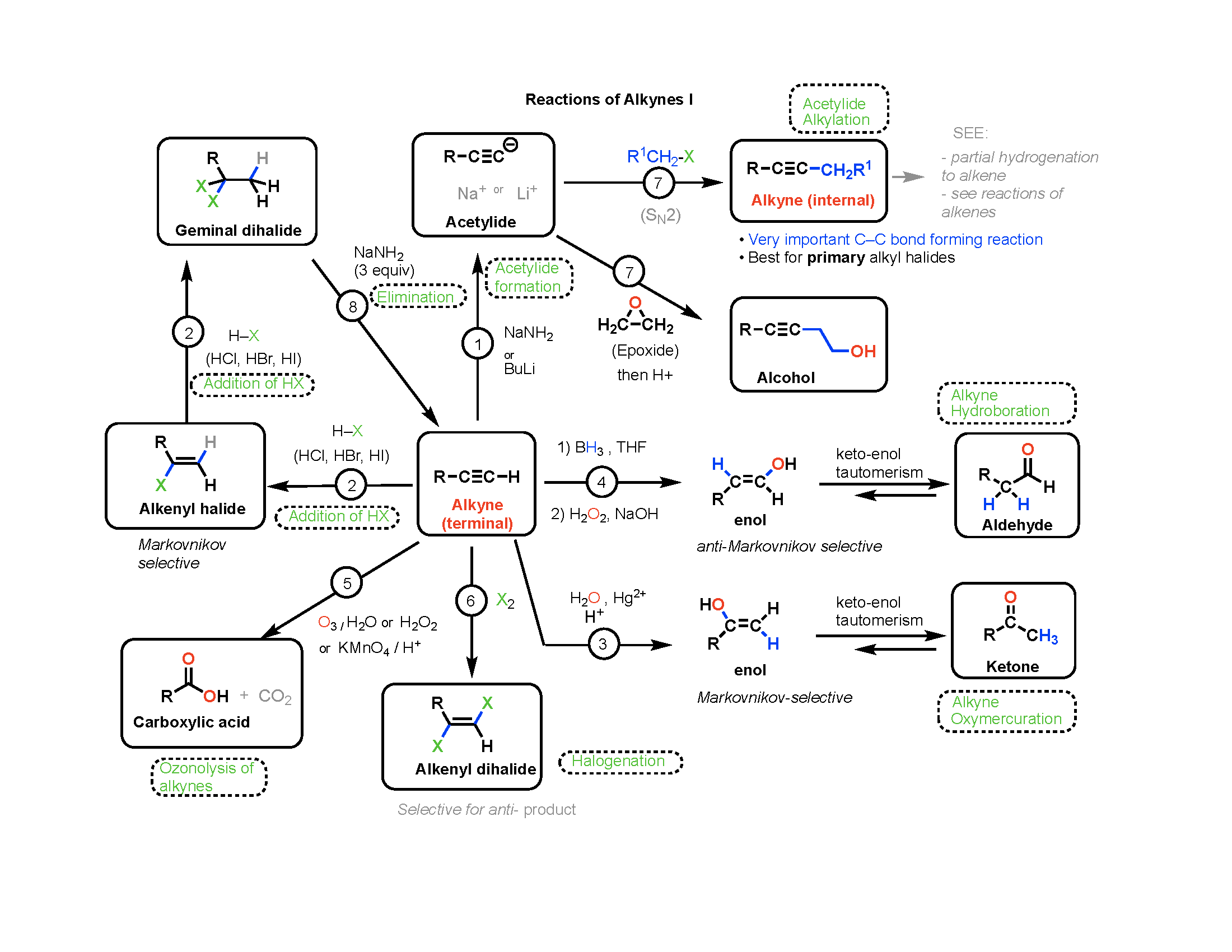
Reaction Maps Now Available Master Organic Chemistry
reaction mechanism, in chemical reactions, the detailed processes by which chemical substances are transformed into other substances. The reactions themselves may involve the interactions of atoms, molecules, ions, electrons, and free radicals, and they may take place in gases, liquids, or solids —or at interfaces between any of these.
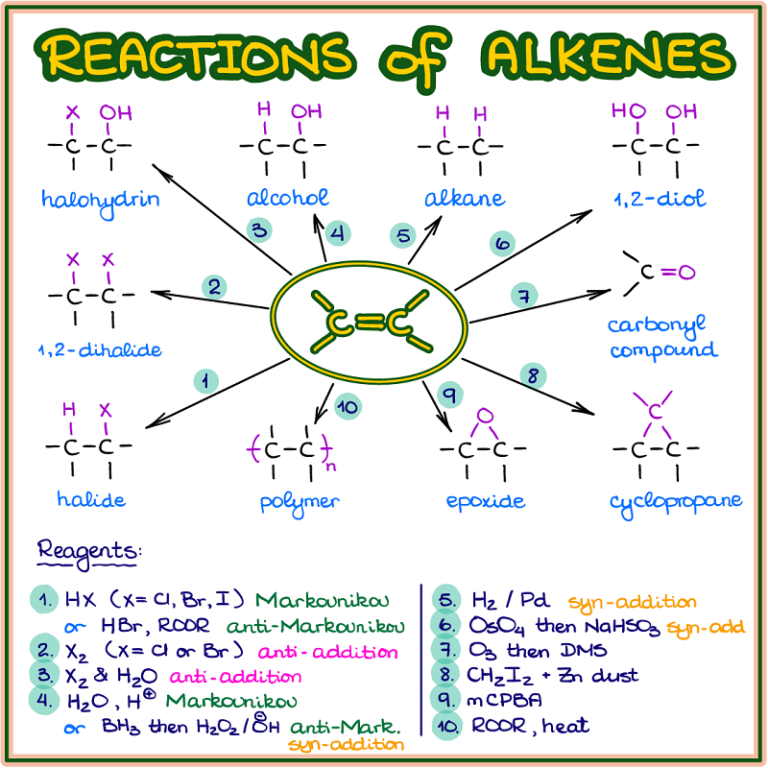
Reactions of Alkenes — Organic Chemistry Tutor
The reaction mechanism (or reaction path) is the process, or pathway, by which a reaction occurs. A chemical reaction usually occurs in steps, although it may not always be obvious to an observer. The decomposition of ozone, for example, appears to follow a mechanism with two steps: